By Peter Graves, P.E., ST Fastening Systems
Background
Corrosion can be compared to an electrochemical reaction where one metal is eaten away in the presence of an electrolyte (moisture and oxygen). The metals are considered dissimilar depending on their position on a galvanic series chart. This chart is arranged in order of their relative electrical potential. The further apart the materials are on the list, the greater the risk of corrosion. As an example, lead acid batteries use this electro-chemical reaction to create electricity, where the dissimilar plates submerged in the battery “acid” create an electron transfer. The ion transfer is from the negative terminal (anode), to the positive terminal (cathode). The anode material “corrodes” sacrificing itself to the cathode. All that is needed is 2 dissimilar metals, and an electrolyte to create this reaction. This test documents the electrical difference between the aluminum panel in question, and the different metals listed when submerged in distilled water, and then again in 5% salt water.
Test Method
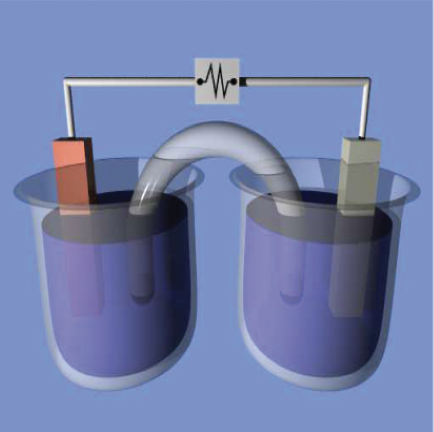
Glass containers were filled with equal amounts of distilled water, and an ion conductor was draped from one container to the other to complete a circuit between the solutions. This ion bridge keeps the solutions separated to limit the cross contamination of the ions dissolved in the container. A test strip of aluminum “panel” was alligator clipped to the negative probe on the multi-meter, one at a time, the list of test pieces shown were clipped to the positive probe of the multi-meter.

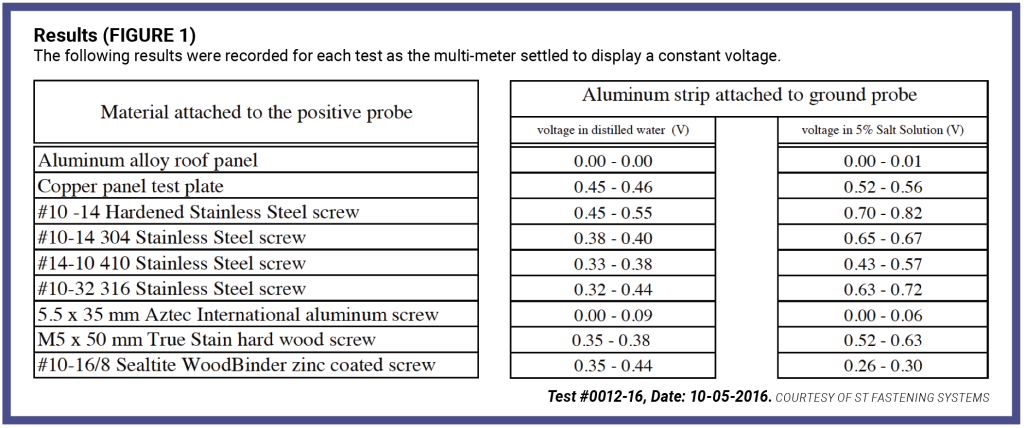
The multi-meter was switched to record DC (direct current) with the aluminum strip submerged in one container, and the tested material were submerged, and the value on the multi-meter was recorded when it reached a consistent value. After each test, the solutions were dumped, the containers washed and refilled for the next comparison. The test was repeated several times and the range of values recorded. [Figure 1, below]
Conclusion
The difference between voltages increased as much as 50% as the electrolytic solution changed from distilled water to salt water. The salt water is a better “electrically conducting solution” due to the salt (NaCl) in the water dissolving into its component ions (Na+ and Cl-). The movement of these negatively charged ions towards the anode, and positively charged ions towards the cathode increases the current flow. The current flow in a salt solution is proportional to the concentration of ions in the solution. The voltage recorded above, shows the smaller the difference in voltage, the more compatible that material is with the aluminum panel. It has been well documented that a metal corrodes quicker the closer it is to marine/ocean environments. In controlled environments, potential difference as great as 0.50 may be acceptable. In moderate environments, metals should have a difference of less than 0.25 volts. In severe coastal environments, the difference in voltage between materials in contact with each other should be below 0.15 volts to minimize corrosion from electro-galvanic reaction. Another factor related to the rate of corrosion of the anode is directly related to the area ratio of the two metals. If the surface area ratio of cathode to anode is doubled, the rate of corrosion of the anode is also doubled, while if the area ratio is halved, the rate of corrosion of the anode is halved.
Aluminum is on the anode side of stainless steel, and the area of the panel compared to the area of the screw is large, so the rate of corrosion would be rapid. Evident with the testing above, aluminum screws are the best solution to use with an aluminum panel, zinc coated carbon screws would react less than all the stainless steel screws tested assuming the zinc coating is not breached during installation. Interestingly the zinc coated screw displayed a lower voltage when tested in the salt solution than in the distilled water.
Summary
To summarize, in severe marine/ocean environments, aluminum fasteners are least likely to be the source of galvanic corrosion from dissimilar metals when fastening aluminum panels to a structure. Consideration of galvanic corrosion of the screw and the substrate should be taken into account also. None of the other fasteners tested fall below the recommended 0.15 volts shown to minimize galvanic corrosive effects in severe conditions. Based on ST’s experience, using carbon steel fasteners with aluminum panels exhibited a possibility that a portion of the galvanized coating will be removed during installation, resulting in an increased risk of dissimilar metal contact. FBN
Peter Graves, P.E., is Vice President of Engineering and Technical Services at ST Fastening Systems.























